
Atom A has a mass number 209 and atomic number 82.
Atom B has a mass number 209 and atomic number 83.
(i) How many protons atom A has ?
(ii) How many protons atom B has ?
(iii) Are atoms A and B isotopes of the same element ?
Atomic Number 82

Atomic Number 82 Element
Solution
Clover for mac osx. Atom A – `'^209'A'_82`
Atom B – `'^209'A'_83`
(i) A has 82 protons as the atomic number is 82.
(ii) B has 83 protons as the atomic number is 83.
(iii) No, A and B are not isotopes of each other because their atomic numbers are not same.
Video TutorialsVIEW ALL [1]

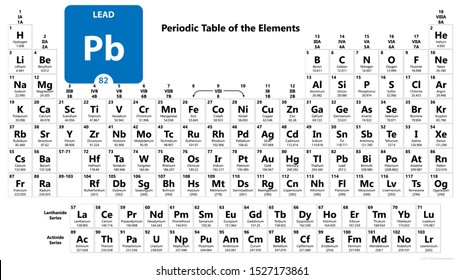
Open firmware for mac. Atomic Number: 82: Atomic Mass: 207.2 atomic mass units: Number of Protons: 82: Number of Neutrons: 125: Number of Electrons: 82: Melting Point: 327.5° C: Boiling Point: 1740.0° C: Density: 11.34 grams per cubic centimeter: Normal Phase: Solid: Family: Other Metals: Period Number. Retina display for mac pro. Atomic Number: 82 Atomic Mass: 207.2 amu Melting Point: 327.5 °C (600.65 K, 621.5 °F) Boiling Point: 1740.0 °C (2013.15 K, 3164.0 °F) Number of Protons/Electrons: 82 Number of Neutrons: 125 Classification: Other Metals Crystal Structure: Cubic Density @ 293 K: 11.34 g/cm 3 Color: bluish Atomic.
view
Video Tutorials For All Subjects
